The CDC just approved the new fall COVID vaccines. Here are some answers to your most pressing questions.
Q: What is different about the updated COVID mRNA vaccines?
The new COVID vaccines are updated versions of the Pfizer and Moderna mRNA vaccines that target the Omicron variant XBB.1.5. These vaccines are designed to provide better protection against this variant and its descendants. Right now, the most common subvariants circulating in the U.S. are EG.5 and FL.1.5.1.
Moderna reported that their updated XBB.1.5 mRNA vaccine caused a significant boost in neutralizing antibodies in humans against EG.5 and FL.1.5.1 that are circulating now. Moderna also protects against XBB.1.5, XBB.1.6, XBB.2.3.2, and BA.2.86 (Pirola).
Pre-clinical data in mice show that the updated Pfizer XBB.1 COVID-19 vaccine generates neutralizing antibodies against multiple circulating Omicron-related sublineages including XBB.1.5, EG.5.1, and XBB.1.16, XBB.2.3 and BA.2.86 (Pirola). Subvariant FL.1.5.1 that is in circulation now was not tested, but is expected to also be susceptible to the Pfizer vaccine.
Novavax is a protein-based vaccine that works differently than mRNA vaccines. Novavax reported that their updated XBB.1.5 COVID-19 vaccine produced a broad neutralizing antibody response against key variants in monkeys. However, the company did not yet have data against FL.1.5.1 or BA.2.86 subvariants. The Novavax vaccine is expected to be FDA approved in the next few months.
Q: Who should get the new COVID vaccines?
The new COVID vaccines are recommended universally for everyone 6 months and older. This includes people who have already been vaccinated with the original Pfizer or Moderna vaccines.
The CDC’s Advisory Committee on Immunization Practices (ACIP) had a meeting on September 12th where they reviewed the data. COVID hospitalizations have increased every week for the last 8 weeks. Hospitalizations, ICU stays and deaths are being seen in healthy people and in those with chronic medical conditions.
Some prior COVID vaccines were approved only for people over age 50. But, with the latest virus variants, data shows that people from age 6 months to adults aged 49 years with no underlying medical conditions are being admitted to the ICU with COVID infections. These younger people need protection from severe COVID infections too.
In addition, half of the COVID deaths in children, while rare, were in kids with no underlying medical conditions. Therefore, it is recommended that all children over age 6 months should get the updated booster vaccine to protect them from severe COVID disease and death.
Although no vaccine protects 100% against a COVID infection, COVID vaccination reduces severe COVID infections and death. COVID vaccines reduce the risk of symptomatic COVID infection in the first few months after vaccination and can make a breakthrough COVID infection milder, shorter or both. COVID vaccines also reduce the risk of getting Long COVID which is a long term disabling disease.
For these reasons, ACIP and the CDC is recommending the fall XBB.1.5 mRNA vaccine boosters for everyone age 6 months and older. The XBB.1.5 mRNA vaccines are safe and effective for people of all ages.
Q: Where can I get the updated XBB.1.5 vaccine?
Many pharmacies and clinics will offer the updated XBB.1.5 mRNA vaccines from Pfizer and Moderna in the next few days. The Novavax vaccine has not been approved yet by the FDA.
To find COVID-19 vaccine locations near you:
Search locations on vaccines.gov
Text your ZIP code to 438829
Call 1-800-232-0233
The new COVID-19 shots will be free to most Americans through private insurance, Medicare or Medicaid. For the uninsured or underinsured, CDC's Bridge Access program will provide no-cost vaccines for adults and the CDC’s Vaccines for Children program will offer them for children.
Q: How many doses of the new XBB.1.5 vaccines will I need?
The number of doses of the new XBB.1.5 COVID vaccine that you should get depends on your age, your medical conditions and your prior COVID vaccination history. Everyone age 5 and older can get a single dose of the updated COVID vaccine, regardless of prior vaccinations or COVID infections.
CDC recommendations for specific groups:
Everyone aged 6 years and older should get 1 updated Pfizer or Moderna COVID-19 vaccine regardless of whether they’ve received any original COVID-19 vaccines.
People aged 65 years and older may get a 2nd dose of the updated Pfizer-BioNTech or Moderna COVID-19 vaccine, 4 or more months after the 1st updated COVID-19 vaccine.
People who are moderately or severely immunocompromised may get additional doses of updated Pfizer-BioNTech or Moderna COVID-19 vaccine 2 months after their 1st dose. Immunocompromised people may require more vaccination doses depending on their particular situation.
Children aged 6 months–5 years may need 1, 2 or 3 doses of the XBB.1.5 COVID-19 vaccine to be up to date, depending on the number of doses they have previously received.
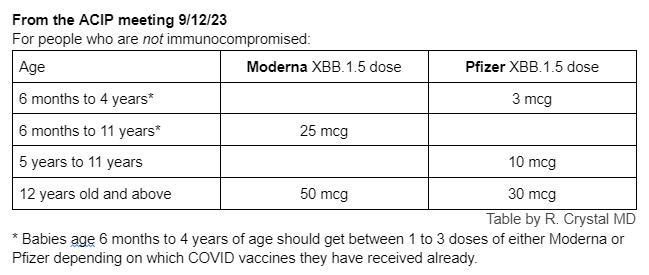
Q: What are the dosages for the XBB.1.5 mRNA vaccines?
Dosages will depend on the person’s age.
Q: My children are under age 5 and not yet vaccinated for COVID. What are the recommendations for them?
Young children under age 5 are immune naive, meaning that their immune system has never had an exposure to either a COVID vaccine or a COVID infection. Therefore, these children need several doses of the COVID vaccine to build lasting immunity.
The American Academy of Pediatrics (AAP) stated that they will have updated COVID vaccine dosing Quick Reference Guide (including cap colors) later this week. (I will add the Quick Reference Guide to this post when it is available.)
9/17/23 The AAP has now posted their Pediatric COVID-19 Vaccine Dosing Quick Reference Guide (aap.org/CovidVaccineGuide)
This CDC slide from the ACIP meeting shows how many vaccine doses are needed for people who do not have immunocompromising conditions:
Adults:
With past mRNA COVID vaccines, people made more neutralizing antibodies with the higher mRNA dose. Since the Moderna vaccine has 50 mcg of XBB.1.5 mRNA for adults and the Pfizer vaccine has 30 mcg of XBB.1.5 mRNA for adults, theoretically, people could make more neutralizing antibodies with the updated Moderna vaccine. However, the difference is probably minimal and it is important to get whichever mRNA vaccine (Pfizer or Moderna) that is available in your area.
Q: When should I get the new COVID vaccines?
According to Megan Wallace at the CDC,
If you were recently vaccinated, you should wait 2 months before getting the updated vaccine.
If you had a recent COVID infection, you can wait 3 months to be vaccinated, or you could get the updated vaccine as soon as you feel better.
If you have not been vaccinated and have not had an infection recently, your immunity may have waned. Since there are many people getting COVID infections now, you may want to schedule your updated XBB.1.5 booster vaccine soon.
Definitely plan on getting a booster by the end of October since a winter COVID surge is expected after holiday get-togethers in November, December, January.
After a COVID infection or vaccination, antibodies last for about 6 months and then their levels decline. This is known as “waning immunity”. A year after a COVID infection or vaccination, neutralizing antibody levels are very low.
Q: What are the side effects of the new COVID vaccines?
The side effects of the new COVID vaccines are similar to the side effects of the original vaccines. The most common side effects are mild and go away on their own within a few days. These side effects may include pain, redness, and swelling at the injection site, as well as fatigue, headache, muscle pain, chills, and fever.
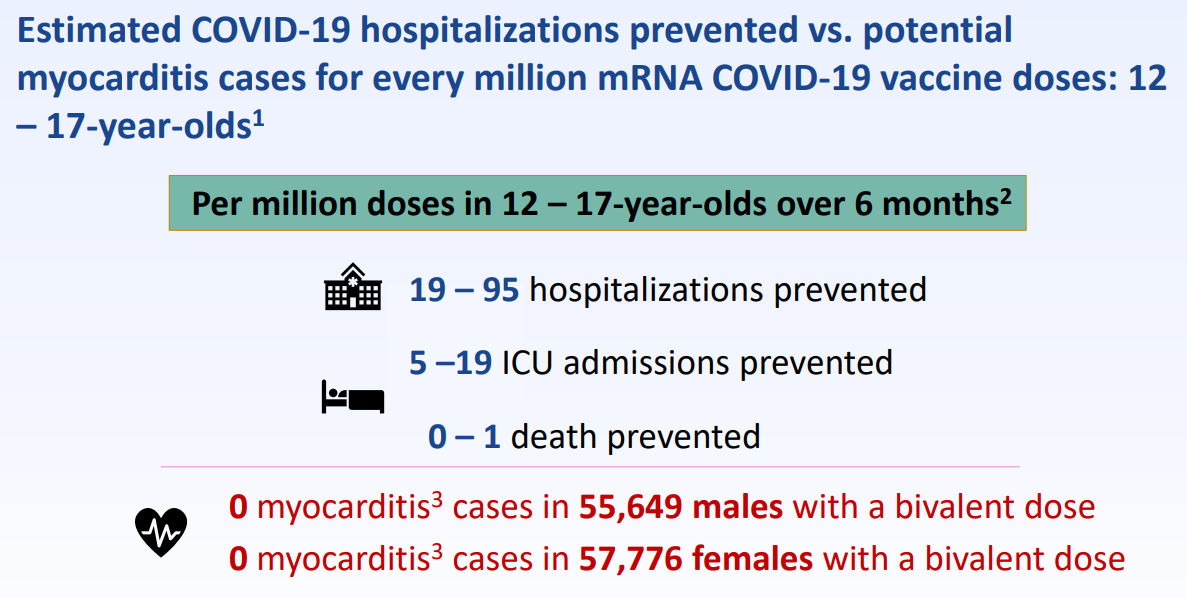
Q: What about the risks of Myocarditis with COVID vaccination especially in young men?
Data shows that the risk of myocarditis decreases with a longer interval between vaccinations. Since it has been a year since many people have had their last booster, this should reduce the risk of myocarditis which is what happened in 2022.
ACIP found that the risk of myocarditis after a booster shot was very low, but the benefits of getting a COVID booster shot for protection against serious illness, hospitalization, and death from COVID far outweigh the risks of vaccine associated myocarditis.
All myocarditis is not equal:
The risk of getting myocarditis from a COVID infection is about 2 to 5 times higher than the risk of getting myocarditis from a COVID vaccination. So it is better to protect oneself against an infection. Also, myocarditis after vaccination tends to resolve without further heart issues, but myocarditis after a COVID infection may cause long term heart problems.
Slide 65
Q: Does it matter in which arm I get the vaccine?
Studies show that it is best to get the booster vaccine in the same arm as previous COVID vaccinations because this produces higher neutralizing antibody levels which is thought to be due to the same draining lymph nodes being stimulated.
Q: Will the new COVID vaccines prevent me from getting COVID-19?
No vaccine is 100% effective, but protection from a COVID infection starts about 2 weeks after getting the updated vaccine and should last a few months. COVID vaccines reduce the risk of symptomatic COVID infection in the first few months after vaccination and can make a breakthrough COVID infection milder, shorter or both.
Protection against severe COVID illness, hospitalization, and death from the current COVID subvariants should last at least 5 to 6 months after vaccination.
COVID vaccines also reduce the risk of getting Long COVID which is a long term disabling disease.
Q: Can I get the updated COVID vaccine and the Flu Shot at the same time?
It is safe to get both the Flu shot and the updated XBB.1.5 mRNA vaccine at the same time. A new study shows that co-administration of the COVID vaccine and Flu vaccine together may lead to minimally lower antibodies (16%) than if vaccinated only with the COVID vaccine, but it is safe to do so and can be much more convenient for many people.
Getting both vaccines at the same time may cause slightly more side effects such as fever, aches and pain at the injection site.
Q: What about the other respiratory viruses in the winter?
This winter, we may have another “tripledemic” of respiratory infections (RSV, Flu and COVID) peaking at around the same time like last year. The respiratory syncytial virus (RSV) season has already started as seen by an increase in RSV cases in the Southeastern United States.
It is recommended that people over age 60 and pregnant individuals receive the new RSV vaccines. (see below)
Q: Can I get the new RSV vaccine at the same time as the updated COVID vaccine?
A newly approved vaccine against respiratory syncytial virus (RSV) is now recommended for people ages 60 and up. There is not enough data yet on giving the new adult RSV vaccine with the COVID booster, so it may be best to give them separately. The RSV season has already started in the southeastern United States.
The FDA also approved a new vaccine for RSV (Abrysvo) to be given to pregnant individuals between 32 and 36 weeks of pregnancy. A single dose of this vaccine in pregnancy helps to prevent lower respiratory tract disease from RSV in infants from birth through 6 months of age.
Q: Should you wait for the Novavax XBB.1.5 vaccine?
Novavax is a protein-based vaccine that works differently than mRNA vaccines. Novavax reported that their updated XBB.1.5 COVID-19 vaccine produced a broad neutralizing antibody response against key variants in monkeys. However, the company did not yet have data against FL.1.5.1 or BA.2.86 subvariants.
The Novavax vaccine is expected to be FDA approved in the next few months, but it is not known exactly when. After FDA approval, Novavax will be available to people age 12 and older.
References:
9/12/23 Slides from the CDC ACIP meeting on September 12, 2023: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/11-COVID-Wallace-508.pdf
9/7/23 MedRxiV: Moderna preprint https://buff.ly/462bqZt
Moderna monovalent XBB.1.5 vaccine causes potent neutralizing responses against variants of the omicron XBB-lineage (XBB.1.5, XBB.1.6, XBB.2.3.2, EG.5.1, and FL.1.5.1) as well as the recently emerged BA.2.86 variant."
9/11/23 Pfizer Press Release: Pfizer and BioNTech Receive U.S. FDA Approval for 2023-2024 COVID-19 Vaccine Pfizer https://buff.ly/45WL8bt
Pre-clinical data show that the updated COVID-19 vaccine generates improved neutralizing antibody responses against multiple circulating Omicron-related sublineages including XBB.1.5, BA.2.86 (Pirola), and EG.5.1 (Eris), which currently accounts for the largest portion of U.S. cases.
9/14/23 Katelyn Jetelina (YLE): Considerations for your fall Covid-19 vaccine https://buff.ly/3RkAOpe
9/13/23 NY Times: What to Know About the New Covid Shots https://buff.ly/3EFkU1k
9/12/23 The Conversation: CDC greenlights two updated COVID-19 vaccines, but how will they fare against the latest variants? 5 questions answered https://buff.ly/3PBkMWS
9/12/23 NY Times: Can You Get the New Covid Vaccine and the Flu Shot at the Same Time? https://buff.ly/3LkKvjN
9/11/23 NBC: FDA clears new Covid boosters: 5 things to know https://buff.ly/3PADSMT
9/8/23 JAMA: Immunogenicity and Reactogenicity of Co-administration of COVID-19 and Flu Vaccines https://buff.ly/3sYYKVe
2/25/22 The Conversation: How long does protective immunity against COVID-19 last after infection or vaccination? Two immunologists explain https://buff.ly/3phpA5V
CDC: Healthcare Providers: RSV Vaccination for Adults 60 Years of Age and Over https://buff.ly/48dfKqL
8/21/23 FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants https://buff.ly/3YMjRpg





As ever, thank you for all you do. I so appreciate your clarity and depth of detail. ❤️