COVID news 11/18/22
Hi all,
BQ.1.(1) is now about 50% of cases and has taken over as the dominant variant from BA.5. Although in vitro lab studies predicted that BQ.1.(1) would be much more immune evasive, so far it appears that clinically we are doing better than expected. Two new studies (Moderna study and study) show that the new bivalent booster protects against BQ.1.(1), XBB and the other new subvariants. Please get a bivalent booster if you haven't already. Any vaccinated person over the age of 5 qualifies for the bivalent booster and boosters have been shown to decrease Long COVID as well.
A new prospective study from Spain followed the course of people who were infected with COVID in 2020. Sixty-percent of hospitalized COVID patients and 68% of non-hospitalized COVID patients still had at least one post-COVID symptom two years after infection. The most common lingering post COVID symptoms were fatigue, pain and memory loss. Patients who had been hospitalized for COVID had more shortness of breath symptoms and those not hospitalized initially had more anosmia (loss of smell) 2 years after COVID infection. Preventative measures including using air filtration, indoor masking when cases increase, doing home antigen tests before a gathering, staying home when sick and getting the new bivalent booster are important to avoid the initial COVID infection and also to prevent long term sequelae.
A prospective cohort study of Paxlovid shows that the rates of excess Paxlovid rebound are actually only 5% rebound seen on positive viral testing and 12% rebound of symptoms. Some control patients who did not take Paxlovid got rebound as well. Although many famous people have had Paxlovid rebound (Dr. Fauci, President Biden, Dr. Walensky), the new study shows that the risks are not that high. Reports on social media can be biased because people who did not get rebound with Paxlovid probably do not post. We need more studies to confirm but it does seem like the risk of Paxlovid rebound is fairly low and at risk people should try to get Paxlovid if they become infected as Paxlovid has been proven to significantly decrease severe disease.
Unfortunately, one of my own family members now has a severe case of Long COVID. While searching for possible treatments, I have noted that there are not many randomized clinical trials reported and although there are some studies that have started, we will not have good data for probably another 1 to 2 years. I have found some interesting information on low dose naltrexone and on PEA (Palmitoylethanolamide) that I have included at the bottom of my article list this week.
I will be taking next week off from the COVID newsletter. I hope that you have a wonderful Thanksgiving holiday.
Take care,
Ruth Ann Crystal MD
Twitter: https://twitter.com/CatchTheBaby
11/18/22
See adorable, extremely rare twin elephants born at Syracuse zoo (photos) https://buff.ly/3TQmCC7
Influenza:
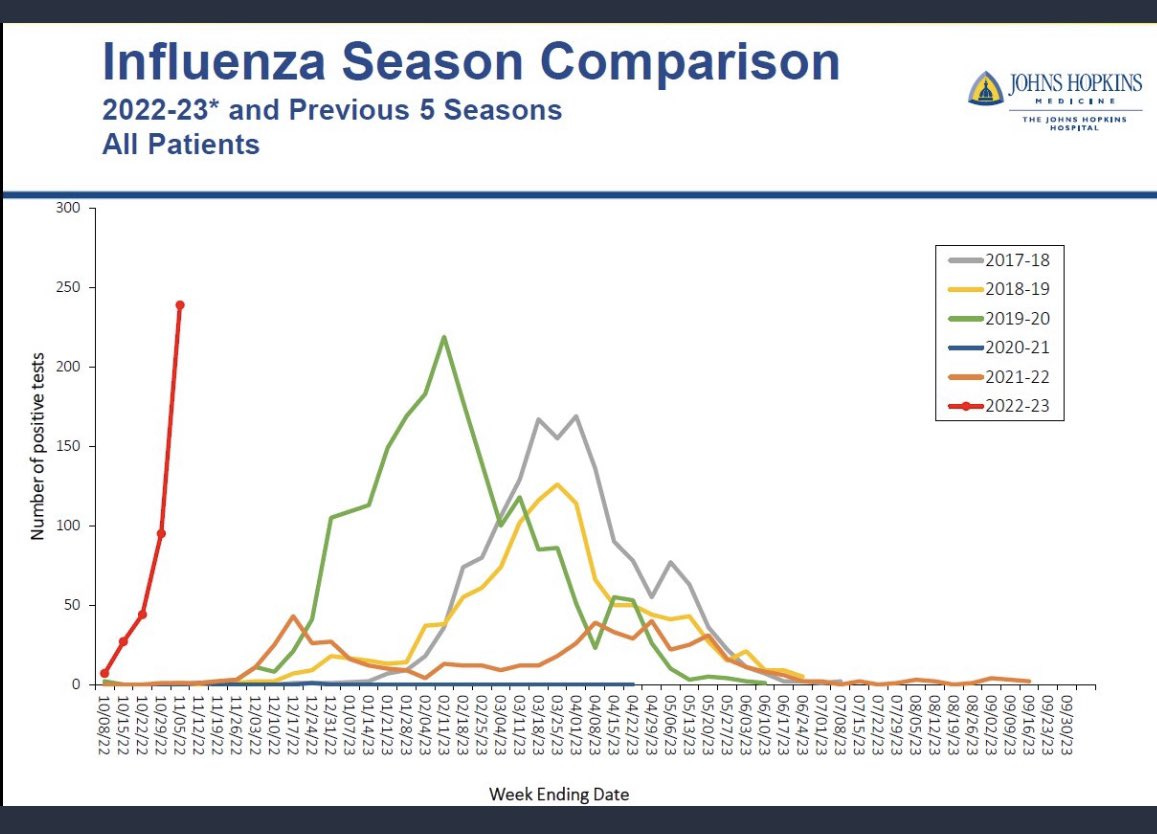
Remember to get your flu shot. The flu is taking off very early and very quickly this year.
_________________
COVID news:
https://newsnodes.com/worldmonitor/
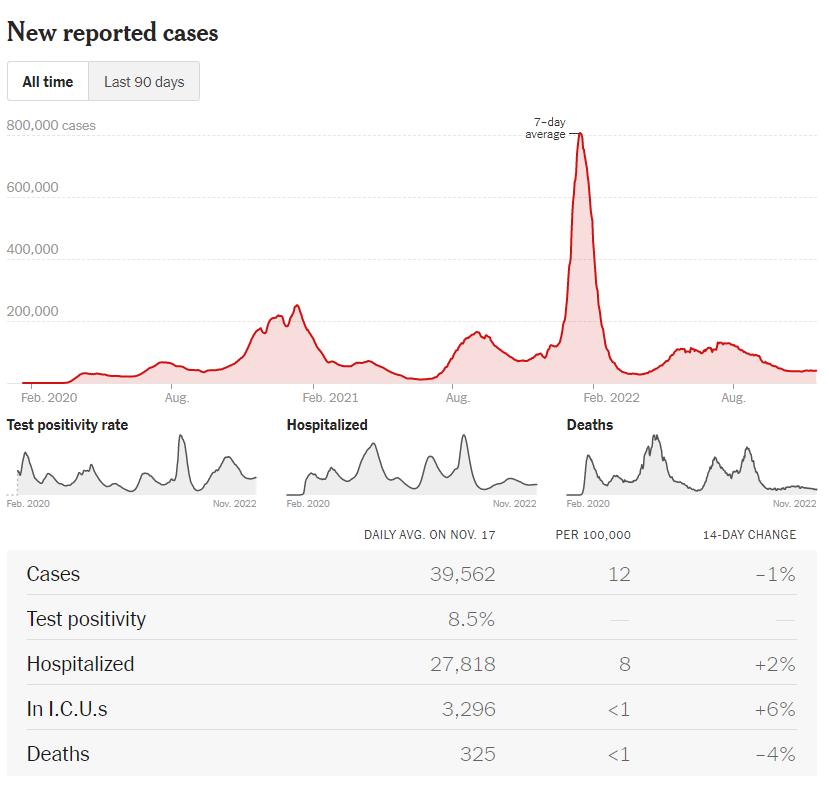
https://www.nytimes.com/interactive/2021/us/covid-cases.html
US cases:
Variant tracker in US: https://covid.cdc.gov/covid-data-tracker/#variant-proportions
BQ.1.(1) is now dominant in the US
Wastewater Monitoring:
CDC Wastewater Monitor https://covid.cdc.gov/covid-data-tracker/#wastewater-surveillance
Biobot: https://biobot.io/data/
Sewer Coronavirus Alert Network (SCAN) project by Stanford University:
Bivalent Vaccine protects better against newer subvariants:
11/17/22 BioRxiV: Improved Neutralization of Omicron BA.4/5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with Bivalent BA.4/5 Vaccine https://buff.ly/3EImlg7
"These data suggest the bivalent BA.4/5 vaccine is more immunogenic than the original ...vaccine against circulating Omicron sublineages, including BQ.1.1 that is becoming prevalent globally."
Figure notation by Eric Topol MD:
11/18/22 Katelyn Jetelina: Who is dying from COVID19? https://buff.ly/3Xf45lO
Vaccines are effective if you’re up-to-date:
Vaccines have saved countless lives. However, it’s increasingly clear that those over 50 must be up to date. Today, 1 in 2 deaths among those over 65 had only one booster. (While ~5% of adults 65 and older remained unvaccinated, they comprised 22% of deaths—a disproportional amount.) Given that only 29% of older adults have their Fall booster means we have a lot of work to do.
11/17/22 MedRxiV: Particulate matter air pollution and COVID-19 infection, severity, and mortality: A systematic review https://buff.ly/3V54qFN
There is strong evidence that ambient PM2.5 increases the risk of COVID-19 infection, and weaker evidence of increases in severe disease and mortality.
11/15/22 Annals of Internal Medicine: Monoclonal Antibodies for Treatment of SARS-CoV-2 Infection During Pregnancy: A Cohort Study https://buff.ly/3UGkFJH
Monoclonal antibody treatment for COVID-19 was safe to use in pregnancy, but it was not very effective.
Patients who were “treated with monoclonal antibodies did not experience differences in obstetric outcomes at delivery...compared to non-treated patients.” However, “there were no statistically significant differences in the composite outcome of COVID-related hospitalizations, emergency department visits, or mortality at 28 days between pregnant patients who did and did not receive treatment.”
11/15/22 JAMA (Spain): Post–COVID-19 Symptoms 2 Years After SARS-CoV-2 Infection https://buff.ly/3EEVzFe
360 hospitalized patients and 308 non-hospitalized patients
59.7% of hospitalized patients and 67.5% of non-hospitalized patients had at least one post–COVID-19 symptom 2 years after infection in 2020.
Hospitalized and non-hospitalized patients ill in 2020 reported 2 years later:
fatigue (44.7% for hospitalized vs 47.7% for non-hospitalized),
pain (5.8%vs 29.9%)
memory loss (20.0% vs 15.9%)
dyspnea (31.1% vs 11.7%; P < .001)
anosmia (10.0% vs 21.4%; P = .003)
Future studies should include uninfected control populations.
11/15/22 MedRxiV: The Paxlovid Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences Between Paxlovid and Untreated COVID-19 Participants https://buff.ly/3tB0fFS
5% rebound by testing: Viral rebound incidence by positive testing was 14.2% in the Paxlovid group and 9.3% in the control group.
12% rebound by symptoms: Symptom rebound incidence was higher in the Paxlovid group (18.9%) compared to the control group (7.0%).
This prospective study suggests that rebound after clearance of test positivity or symptom resolution is higher than previously reported. However, we observed a similar rate of rebound in both the Paxlovid and control groups.
11/16/22 Paxlovid rebound: new data reviewed https://buff.ly/3ObxzNc
More studies need to be done.
We can’t rely on perceptions based on anecdotes and social media. There is a bias of ascertainment and reporting, since the people who did well with Paxlovid, without rebound, are likely to be quiet, no less the people who did not take Paxlovid but later developed positive tests, with or without symptoms. The only way to know is to conduct a prospective, systematic clinical trial, with controls, which our group did, to shed light on the frequency of this Paxlovid problem.
The excess in rebound with Paxlovid versus controls is low, 5-12% for viral testing rebound and symptomatic rebound, respectively. That is considerably less than our expectations and some estimates shared that were well over 50%. This should be reassuring to prescribers (which include pharmacists) and patients, that the chances of rebound are low. The empiric need to take longer duration of Paxlovid (i.e. 7-10 days) is reduced from these findings, but certainly that strategy should be further explored, especially if there is a way to know a priori which individuals would be at risk for rebound and might benefit from a longer course.
11/14/22 Eric Topol MD: New booster data and variants galore https://buff.ly/3ganDHo
New bivalent booster data from Moderna shows good nAb response to BA.4/5 and "robust" nAb to BQ.1.1
Optimism about newer subvariants like BQ.1.1 being not as worrisome as predicted by in vitro studies.
We need to be careful as people gather indoors during the holidays.
11/14/22 STATnews: Moderna data suggest new bivalent Covid booster is more effective against Omicron variants https://buff.ly/3THdQpZ
n > 500 adults
Bivalent Moderna booster:
15x neutralizing antibodies (nAbs) to BA.4 and BA.5
5x to 6x higher BA.4/BA.5 nAbs for new bivalent booster compared to original Moderna booster.
Moderna's new bivalent booster appeared to be effective (‘robust’) against BQ1.1, in a preliminary study using samples from 40 of the study’s volunteers.
11/13/22 Washington Post: The latest coronavirus variants show reassuring early signs https://buff.ly/3Ag7M0u
Via Eric Topol
11/14/22 Nature Metabolism: COVID-19 and Diabetes — where are we now? https://buff.ly/3Ag2BxN
Covid has been associated with an increased risk of both Type 1 (autoimmune) and Type 2 diabetes.
Review of possible mechanisms.
11/11/22 Clinical Neurophysiology: Co-Ultramicronized Palmitoylethanolamide/Luteolin (PEA-LUT) normalizes GABA B-ergic activity and cortical plasticity in Long COVID syndrome https://buff.ly/3Gdt2I8
N = 39 patients randomized
Patients were assigned to one of two study groups (n=17 each), receiving either PEA-LUT (Glialia®, 700 mg + 70 mg, sublingual microgranule formulation, Epitech Group SpA, Saccolongo, Italy) or PLACEBO (sublingual inert microgranules), administered orally bid for eight weeks. Glialia® is licensed in Italy as an oral food product for special medical purposes, with anti-inflammatory and neuroprotective properties.
Long Covid patients with fatigue and cognitive problems show impairment of cortical GABA B activity and reduced plasticity in primary motor cortex.
Co-ultramicronized palmitoylethanolamide with luteolin (PEA-LUT) 700+70 mg bid for 8 weeks restores GABA B neurotransmission and cortical plasticity.
PEA-LUT is a candidate for the treatment of long Covid patients.
10/24/22 Brain Behav Immune Health: Safety and efficacy of low dose naltrexone in a long covid cohort; an interventional pre-post study https://buff.ly/3AenTMi
n = 38, 35% HCW
Improvement was seen in 6 of 7 parameters measured on QoL questionnaire: recovery from COVID-19, limitation in activities of daily living, energy levels, pain levels, levels of concentration and sleep disturbance (p ≤ 0.001), improvement in mood approached but was not significant (p = 0.054).
10/18/22 Reuters: Addiction drug low dose naltrexone (LDN) shows promise lifting long COVID brain fog, fatigue https://buff.ly/3SiPgLs
Good summary
LDN may repair damage of the disease rather than mask its symptoms.
There are now at least four clinical trials planned.
Dr. Hector Bonilla, co-director of the Stanford Post-Acute COVID-19 Clinic and a RECOVER adviser, has used LDN in 500 ME/CFS patients, with about half reporting benefits.
He studied LDN in 18 long COVID patients, with 11 showing improvements, and said he believes larger, formal trials could determine whether LDN offers a true benefit.
10/13/22 MedRxiV (Douglas B Kell, Etheresia Pretorius):
Increased levels of inflammatory molecules in blood of Long COVID patients point to thrombotic endotheliitis https://buff.ly/3Gt0URk
Microclotting, thrombotic endotheliitis in Long COVID.
Von Willebrand Factor, platelet factor 4,serum amyloid A, α-2antiplasmin E-selectin, and platelet endothelial cell adhesion molecule-1
11/11/22 Healio Rheumatology by Leonard H. Calabrese, DO:
Long COVID and Me: A True Story https://buff.ly/3fZg5an
A doctor with Long COVID after a breakthrough infection in 2021 what it was like for him to have brain fog and tachycardia.